


Pharmacovigilance has historically focussed on the post-authorisation period. However, as the science and the legislation have evolved, pharmacovigilance has rightly moved towards a proactive, as opposed to a reactive, consideration of risks. This has led to ever more sophisticated risk management systems.1
A risk management plan (RMP) was first introduced into pharmacovigilance in 2005. In 2012, along with the new legislation, a new format of the RMP was introduced, as well as GVP Module V.2 As per the module, an RMP details the known concerns about the safety of the medicine and how they can be managed. The RMP may also outline any additional studies that have been recommended at the time of marketing authorisation (MA) approval to provide more information on the medicine’s safety profile. It is submitted as part of the dossier that is evaluated by regulatory authorities before a medicine can be authorised.3
March 2017 saw the first revision of the GVP module since its introduction; hoped to reflect lessons learned by industry over the previous five years and refine the process to minimise repetition. The long-awaited revision has been anticipated to provide clarification on the focus of RMPs and a more pragmatic approach to RMPs for generics.
With the revised module, comes the revised EU template for RMPs. Upon its publication, the European Medicines Agency (EMA) introduced transitional arrangements regarding the use of the template. This states marketing authorisation holders and applicants can use the new template for all RMP submission as of 31 March 2017. However, its use does not become mandatory for all RMP submissions until 31 March 2018.5
As described by the EMA, the revised GVP modules contain “major revisions”.4 These are most clearly reflected in the template update. On the face of it, this now appears to offer much more detailed guidance to applicants on what information is required in each section of the RMP. The RMP template now contains a “General considerations and guidance” section at the beginning. This includes a “Checklist for writing or assessing an RMP”. It is possible the regulators envisage this being used as a “self-QC” by applicants, prior to submission of the RMP, to prevent common omissions. The checklist informs generic or hybrid applications, to include all safety concerns from the latest version of the RMP for the reference medicinal product or from a list of safety concerns published on the CMDh website.5 The other main differences can be seen in the below two summary tables:
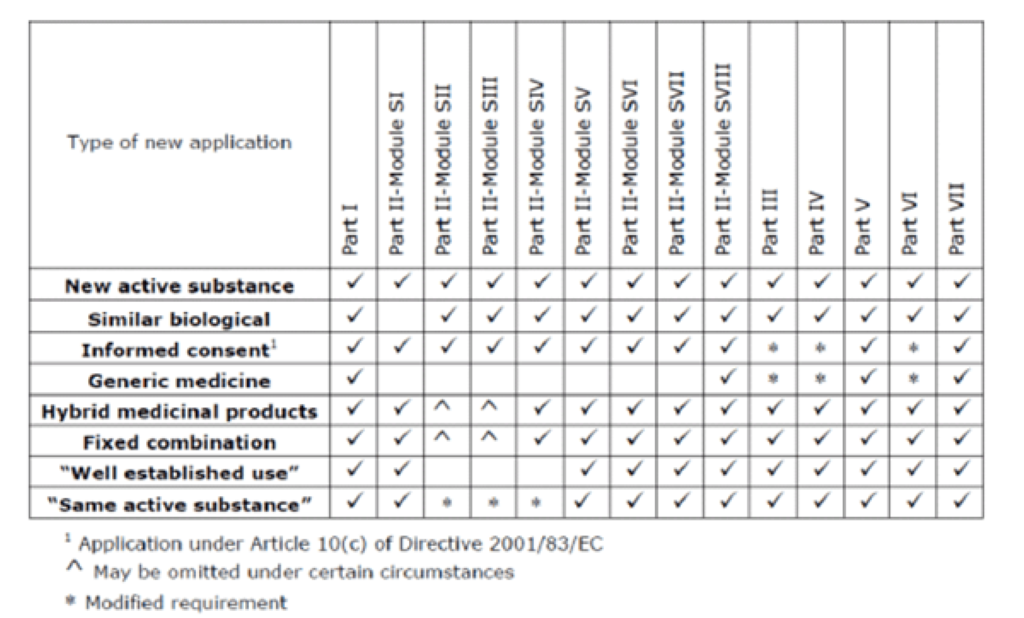
a. Revision 1 GVP Module V minimum requirements2
b. Revision 2 GVP Module V minimum requirements4
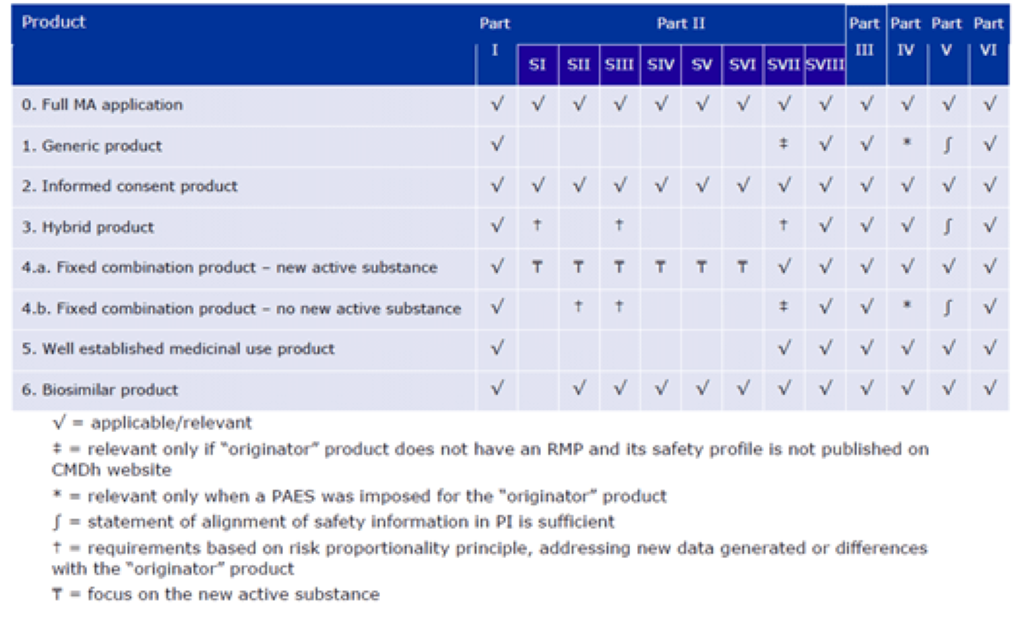
Of note, hybrid products and well-established use products are now required to include a lot less detail in the RMP. Furthermore, fixed combinations products have been divided into those that have a new active substance and those that do not, and this dictates the content of the RMP. Generics are required to complete largely the same sections as previously, however, certain statements are now permitted in lieu of completing the full section. For example, Part II: Section SVII is only relevant if the originator does not have an RMP or the safety profile is not published on the CMDh website. Part IV is now only relevant when a post authorisation efficacy study (PAES) is required for the originator. Finally, most notably, Part V, which previously contained all the sections of the SmPC and PIL relevant to the product safety concerns, may now contain a statement that the safety information is in line with the originator. At Panacea, we often prepare RMPs for generic products, and as such this risk proportionate approach to writing RMPs is welcomed.
Another major update to the template concerns the public summary of the RMP. In March 2014, the Agency began publishing summaries of RMPs for centrally authorised medicines. These are intended to allow stakeholders wider access to the information behind the decision-making process of European regulatory authorities when they review the safety of a medicine or active substance.3 However the sections of the public summary of the old RMP template did not align with what was included in the EPAR, and furthermore, as apparent from the title of this section, it should be a summary of what has been included in the main sections, however, it was unclear for applicants what sections to draw parallels with and sometimes, new information was included here instead. The new template rectifies this by providing much clearer guidance.5
In conclusion, the updates made to GVP Module V and the EMA RMP template seem to be sensible and helpful to applicants, and prevent unnecessary work by applicants where hybrid, generic and fixed combination products with no new active substance are concerned. The only potential disappointment concerns the update to GVP Module XVI – Risk minimisation measures: selection of tools and effectiveness indicators. This has only been updated to reflect changes to the aforementioned Module V, and does not provide any clearer guidance on measuring effectiveness of risk minimisation measures. It is hoped guidance that will differentiate between the abilities of Big Pharma and smaller generics companies will be provided for this in the future.
For more information, or to request a proposal for an RMP, please e-mail: bd@panacea.im
References