


by Lauren Morley, Senior Regulatory Affairs Specialist at Panacea
Those in the regulatory affairs field will remember how arduous and time-consuming that task of completing paper application forms for new Marketing Authorisation Applications was. Many will also remember that this task was prone to transcription errors as the information was added manually and came from various sources and in multiple dossier sections.
In more recent years, the introduction of the electronic application forms (eAF) in the EU has made the task of completing application forms less time-consuming and has reduced the risk of transcription errors. The use of eAFs became mandatory in July 2015 for the centralised procedure and in January 2016 for all MRP/DCP and national procedures.
The use of eAFs included new possibilities like the electronic data import/export function, data population within the form, online access to standardised catalogue terms and built-in business rule validation. With the phased implementation of ISO IDMP and the use of master data in the eAFs, there has been a move to standardised terminology.
Since the introduction of the eAFs, a further improvement was the Digital Application Dataset Integration (DADI) Network Project which was launched in December 2020. The project aims to replace the current PDF-based electronic application forms (eAFs) with new web-based forms. Recently, reference to the DADI project has been phased out in favour of the term eAF Product.
This project is not a new concept, as work on replacing the electronic application forms was first undertaken as part of the Common European Single Submission Portal (CESSP) Phase 1 project which initially started in 2016. This phase was to initially deliver web-based application forms for MAAs for human and veterinary domains, followed by subsequent releases of variation and renewal forms. However, the project faced implementation challenges and budgetary constraints and CESSP Phase 1 project was stopped in October 2020.
The eAF Product will change the input of data into the forms from the PDF-based application to web forms. However, this will not change the output of the data, which will remain as a PDF. The process to submit MAAs will not change, nor will the content of the application form in the submission.
The changes are as follows:
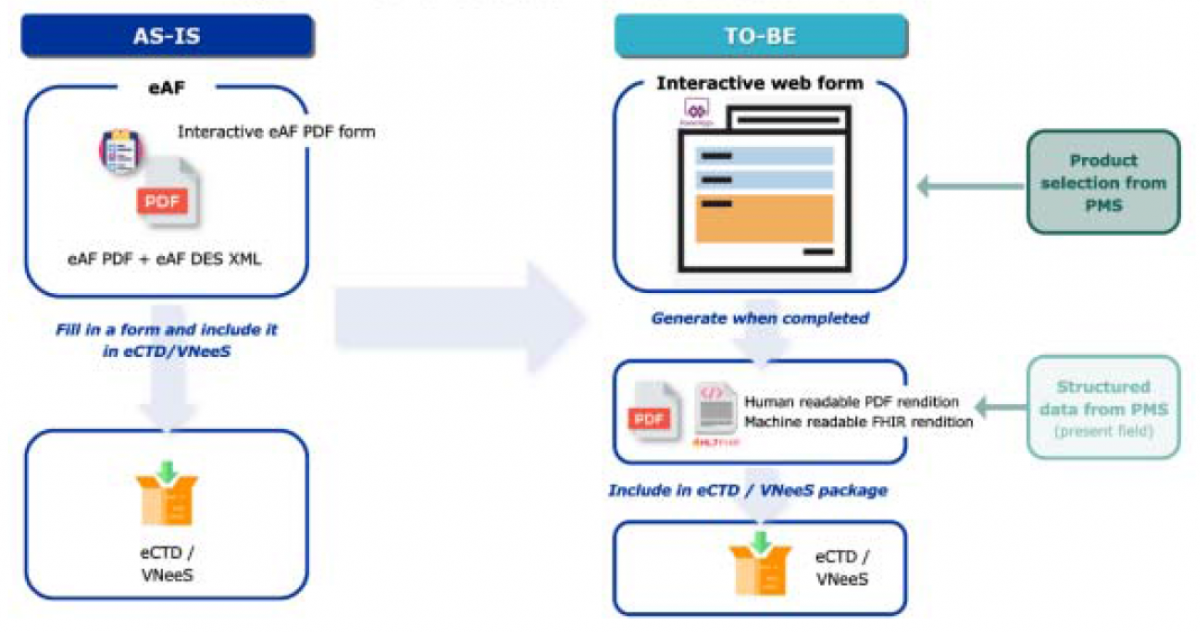
FHIR (pronounced as ‘fire’) stands for Fast Healthcare Interoperability Resources, which is a standard for health care data exchange. It is a machine format that can be read and processed by IT systems. The FHIR message will provide standard PMS data, and will be used to support the exchange of data with product databases such as PMS, thereby ensuring ISO IDMP compliance.
ISO IDMP will be implemented in phases through a set of projects known collectively as SPOR. These are:

The PMS will contain product data taken from a variety of databases in the EU, such as SIAMED (for centrally authorised products) and Article 57/xEVMPD (for both centrally authorised and nationally authorised products). The PMS will eventually replace xEVMPD. For more details on ISO IDMP and the implementation of PMS, see Substance and product data management services on the EMA website.
The new web-based eAFs will use data from the PMS, so in order for the EMA to deliver the eAF Product, product data needs to be migrated from SIAMED and xEVMPD. The migration of data is currently ongoing, as of July 2023.
The eAF Product will first replace the variations application form for human medicinal products, followed by other submissions forms for centrally and nationally authorised products. The Product Lifecycle Management (PLM) portal will host all web based eAFs.
Industry has already seen the release of the new web-based human variations eAF in November 2022. This was the start of the optional use of the variations eAF for centrally authorised products (CAPs) only. Following this, the target date for the start of the optional use of the variations eAF for nationally authorised products (NAPs) is currently expected between November 2023 and February 2024. There will be a 6-month transition period until the use of the web-based variations eAF will become mandatory for both CAPs and NAPs.
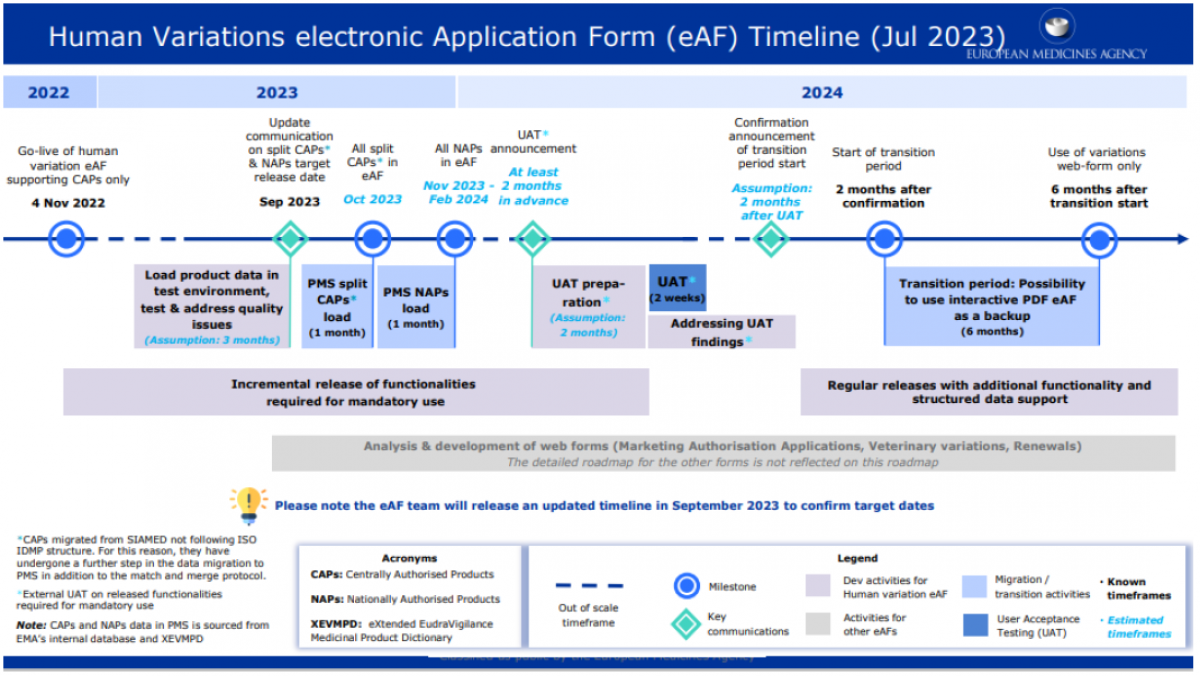
Other web-based eAFs for human and veterinary marketing authorisation applications, veterinary variations and human renewals are also planned, but the timelines for these are not yet known.
Once the EMA has delivered the web-based eAFs, it will form part of EMA’s Regulatory Business Optimisation, which aims to transform processes using digital technology. This will eventually integrate eAF and data submission on authorised medicines submissions into a single process – which is known as the PMS Target Operating Model. The TOM will allow product data to be re-used in regulatory processes and applications.
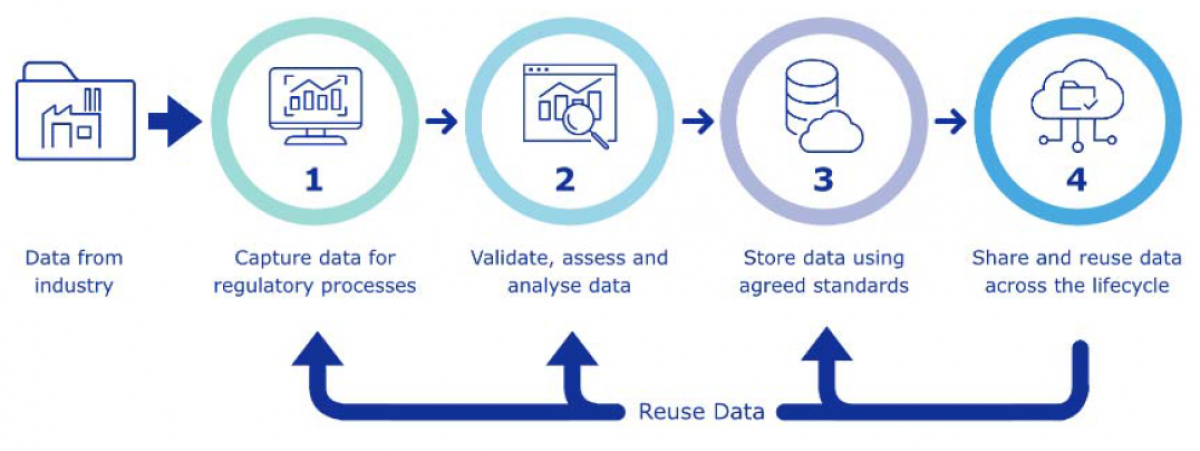
This will facilitate greater efficiency, and better data quality overall. For Industry, it remains to be seen whether the implementation of the web-based eAFs and the PMS TOM will provide similar benefits in terms of time-saving and accuracy of data in regulatory activities in the future.